Part D. Chapter 5: Food Sustainability and Safety
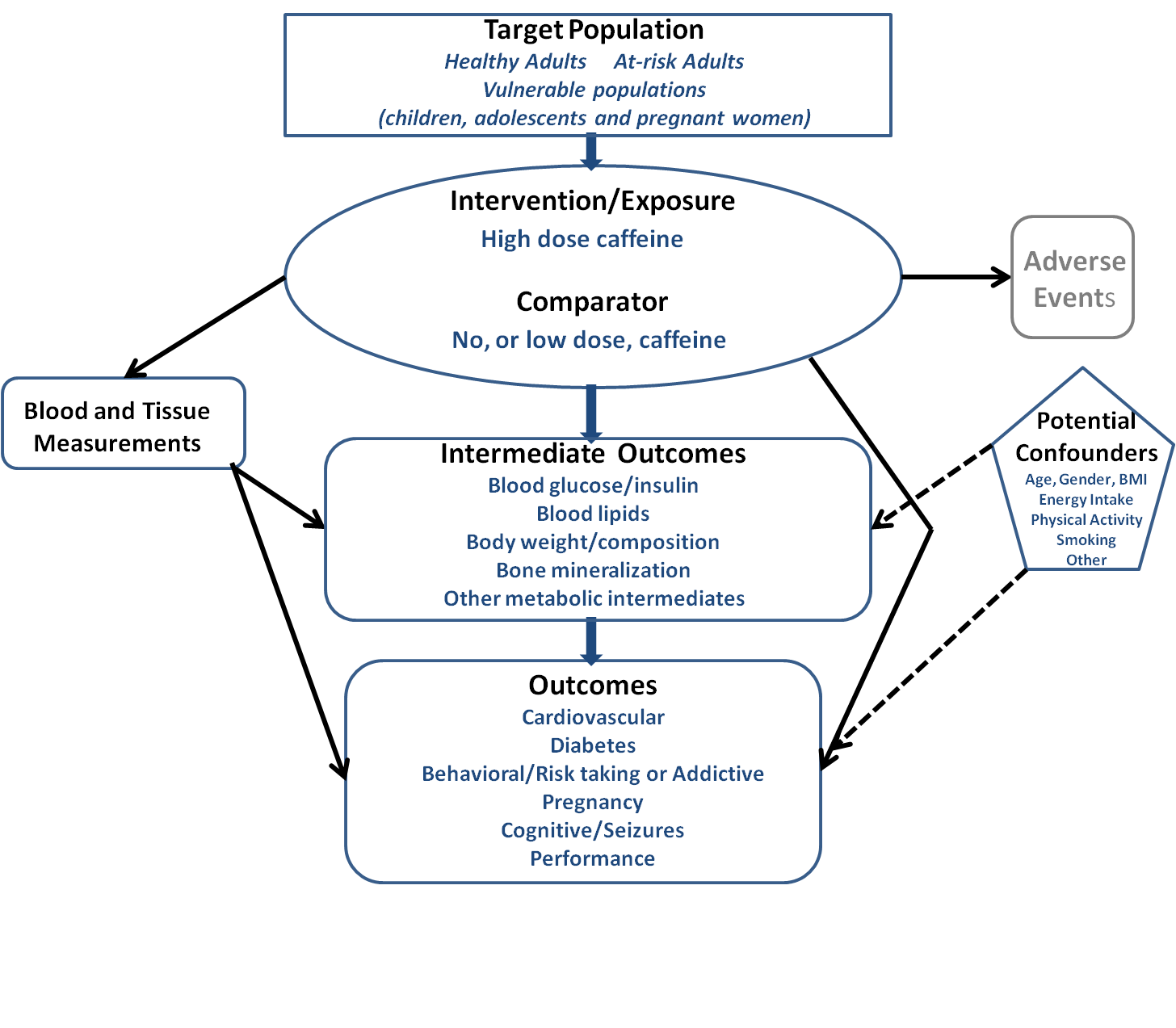
What is the relationship between high-dose caffeine consumption and health?
Conclusion Statement: Evidence on the effects of excessive caffeine intake on the health of adults or children (>400 mg/day for adults; undetermined for children and adolescents) is limited. Some evidence links high caffeine intake in the form of energy drinks to certain adverse outcomes, such as caffeine toxicity and cardiovascular events. Randomized controlled trials (RCTs) on the relationship between high-caffeine energy drinks and cardiovascular risk factors and other health outcomes report mixed results. Evidence also is limited on the health effects of mixing alcohol with energy drinks, but some evidence suggests that energy drinks may mask the effects of alcohol intoxication, so an individual may drink more and increase their risk of alcohol-related adverse events.
DGAC Grade: Limited
Key Findings
- Early safety signals consisting of case reports of adverse events associated with high-caffeine drink consumption, including increased emergency room visits, indicate a potential public health problem.
- The DGAC agrees with the American Academy of Pediatrics and the American Medical Association that until safety has been demonstrated, limited or no consumption of high-caffeine drinks, or other products with high amounts of caffeine, is advised for vulnerable populations, including children and adolescents.
- High-caffeine energy drinks and alcoholic beverages should not be consumed together, either mixed together or consumed at the same sitting. This is especially true for children and adolescents.
Background
According to the FDA, the upper limit of moderate caffeine intake in healthy adult populations (barring pregnant women) is 400 mg/day, with intakes higher than this being considered excessive caffeine consumption. The FDA has not defined moderate and excessive intake levels for children and adolescents. However, according to Health Canada, children should not consume more than 2.5 mg of caffeine per kg bodyweight per day (http://www.hc-sc.gc.ca/fn-an/securit/addit/caf/food-caf-aliments-eng.php ). While this guideline only pertains to children up to the age of 12 years, in the literature it is usually applied to children and adolescents of all ages. A caffeine threshold of 2.5 mg/kg/day would translate into around 37.5 mg/day for 2-5 year olds with an average weight of 15 kg, 75 mg/day for 6-12 year olds with an average weight of 30 kg, and 137.5 mg/day for 13-17 year olds with an average weight of 55 kg.
The main sources of caffeine among both adults and children are coffee, tea, and carbonated soft drinks. Another product, which has received a lot of attention recently as a potential source of excessive caffeine intake, especially among younger populations, is energy drinks (Pomeranz et al, 2013). An energy drink is a beverage that contains caffeine as its active ingredient, along with other ingredients such as taurine, herbal supplements, vitamins, and sugar. It is usually marketed as a product that can improve energy, stamina, athletic performance, or concentration (Seifert et al, 2011). Energy drinks have recently evaded oversight and regulation due to their variable, sometimes excessively high caffeine content (from 50 to 505 mg per can/bottle, with caffeine concentrations anywhere between 2.5 to 171 mg per fluid ounce) (Reissig et al, 2009), which is not regulated by the FDA due to the classification of energy drinks as dietary supplements (Seifert et al, 2011).
Health organizations including the American Academy of Pediatrics, the International Society of Sports Nutrition, and the American Medical Association have issued position statements on energy drinks, advising limited/no consumption among children and adolescents. Given the increasing evidence pointing towards harmful effects of excessive caffeine consumption, the FDA requested the Institute of Medicine (IOM) convene a workshop examining the science behind safe levels of caffeine intake. A report summarizing this workshop was recently published (Institute of Medicine, 2014). Its main conclusions were: 1) Children and adolescents are a potential vulnerable group, in whom caffeine intake could have detrimental health consequences. This is particularly important given insufficient data on caffeine consumption in this demographic, which is increasingly getting exposed to new modes of caffeine intake such as energy drinks; 2) Not enough is understood about potential interactions between caffeine and other ingredients commonly found in caffeine containing foods and beverages; and 3) More research is needed on identifying individual differences in reactions to caffeine, and vulnerable populations, including children with underlying heart conditions, and individuals with genetic predispositions to heart conditions.
The Center for Disease Control (CDC) recently reported on trends in caffeine intake over the past decade (1999-2010) among US children, adolescents, and young adults (Branum 2014). The CDC found that although energy drinks were not widely available prior to 1999, energy drinks made up nearly 6% of caffeine intake in 2009–2010, indicating fast growth in US consumption over a short period of time. When energy drink consumption was assessed in a nationally-representative sample of US secondary school students (Terry-McElrath 2014), 35% of 8th graders, 30% of 10th graders, and 31% of 12th graders consumed energy drinks or shots, and consumption was higher for adolescent boys than girls. Furthermore, energy drink use was associated with higher prevalence of substance use, as assessed for all grades of US secondary students.
Furthermore, a serious issue of public health concern has been the popular trend of combining energy drinks with alcoholic beverages. In 2010, the FDA determined that caffeine added to alcoholic beverages was not generally recognize as safe (GRAS), leading to withdrawal of premixed, caffeinated alcoholic beverages from the market (Arria and O’Brian 2011). Currently, Health Canada caps caffeine levels for energy drinks at 100 mg/250 ml (~1 cup) and has determined that an energy drink container that cannot be resealed be treated as a single-serving container. They have also mandated that manufacturers add a warning to labels that energy drinks should not be combined with alcohol. Recently, the CDC has made public statements on the dangers of mixing alcohol and energy drinks. They indicate that high amounts of caffeine in energy drinks can mask the intoxicating effects of alcohol, while at the same time they have no effect on the metabolism of alcohol by the liver. Therefore, high amounts of caffeine in energy drinks may result in an “awake” state of intoxication, thus increasing the risk of alcohol-related harm and injury (http://www.cdc.gov/alcohol/fact-sheets/cab.htm, March 2014).
Description of the Evidence
Several case reports of adverse events related to energy drink use have been published. A recent systematic review of case reports of adverse cardiovascular events related to consumption of energy drinks documented 17 such published case reports (Goldfarb et al, 2014). The cardiovascular events so documented included atrial fibrillation, ventricular fibrillation, supraventricular tachycardia, prolonged QT, and ST elevation. In 41% of the cases, there had been heavy consumption of energy drinks, and 29% of the cases were associated with consumption of energy drinks together with alcohol or other drugs. In 88% of the cases, no underlying cardiac condition was found which could potentially explain the cardiovascular event, although there was co-occurrence of other cardiovascular risk factors along with energy drink consumption prior to onset of the event in most cases. Of the cases that presented with serious adverse events, including cardiac arrest, the majority occurred with either acute heavy consumption of energy drinks or in combination with alcohol or other drugs. Overall, the authors concluded that causality cannot be inferred from this case series, but physicians should routinely inquire about energy drink consumption in relevant cases and vulnerable consumers should be cautioned against heavy consumption of energy drinks or concomitant alcohol (or drug) ingestion. This systematic review is consistent with a recent report from the Drug Abuse Warning Network (DAWN) on energy drink-related emergency room visits that showed US emergency room visits temporally related to energy drink consumption doubled from 2007 – 2011 (http://www.samhsa.gov/data/2k13/DAWN126/sr126-energy-drinks-use.pdf [PDF - 819 KB]). These visits were attributed mainly to adverse reactions to energy drinks, but also to combination with alcohol or drugs. It is generally agreed that adverse events associated with energy drink consumption are underreported.
Several short-term randomized controlled trials (RCTs) have examined the health effects of energy drink consumption. All of these have been carried out in adult populations, probably due to ethical constraints in providing energy drinks to children. Burrows et al (2013) recently published a systematic review of RCTs examining this question. They found 15 such RCTS, examining the effect of variable doses of energy drinks (mean dose: one and a half 250ml cans per study session) with differing ingredient combinations and concentrations on a number of different health outcomes. The high variability in exposure and outcome definitions made a meta-analysis infeasible. Overall, they found no consistent effects of energy drinks on cardiorespiratory outcomes (heart rate, arrhythmias, blood pressure), pathological outcomes (blood glucose, blood lactate, free fatty acids, clinical safety markers), and body composition, with some studies showing positive, some inverse, and some no associations. For many of these outcomes, consistent results could not be stated due to only one study reporting on them. There was a slight indication of a potential positive effect of energy drinks on physiological outcomes (run time to exhaustion, peak oxygen uptake, resting energy expenditure); however the authors concluded that more studies were needed before arriving at a definitive conclusion. Two of the studies assessed the simultaneous ingestion of alcohol and energy drinks (Ferreira 2006; Wiklund 2009). One found that when compared with the ingestion of alcohol alone, the addition of an energy drink reduced individuals’ perception of impairment from alcohol, while at the same time, objective measures indicated ongoing deficits in motor coordination and visual acuity (Ferreira 2006). Nor did energy drinks reduce breath alcohol concentration, indicating no change or increase in alcohol metabolism by the liver. Another study on energy drinks in combination with alcohol and exercise showed that during post-exercise recovery there was no effect on arrhythmias within 6 hours of energy drink ingestion in healthy young adults (Wiklund 2009).
There are several issues with many of the above studies, such as lack of a true control group (water or no drink), a very short follow-up duration of only a few hours, and small sample sizes, which could explain the inconsistent findings. In addition, many of these studies did not report whether they were commercially funded. Several of those that did report funding sources had financial conflicts of interest. Lastly, the doses of energy drinks used in these studies were not too high, resulting in caffeine intake levels that fell within the normal range. It is possible that excessive caffeine intake due to heavy energy drink consumption adversely impacts several health outcomes, but this hypothesis was not clearly addressed by these studies. Hence it is difficult to ascertain the impact of excessive caffeine intake on health outcomes on the basis of these RCTs. There is also very little data on the health effects of excessive caffeine consumption in pediatric populations.
Table 1. Summary of Studies on High-dose Caffeine Consumption and Health
|
Author, Year Risk of Bias Study Design |
Location Duration |
Sample |
Intervention/ Exposure |
Results |
|---|---|---|---|---|
|
Burrows et al., 2013 AMSTAR: 7/11 Systematic Review: 15 intervention trials 5 RCTs; 10 Pseudo-RCTs (alternate allocation or other method) No overlap w/ Goldfarb 2014 (case reports) |
5 in US; 4 UK, 2 Germany, 1 in Canada, Brazil, Sweden, and Australia Trial duration: Short-term: 30 min-3 h Long-term: 4-10 wk |
Range N = 10 - 69 (mostly crossover) Mean N = 25 32-70% Women; 3 trials all Men Mean Age = 25y; Range = 18-45y |
10 studies used standard Cal EDs; 6 studies used low-Cal or sugar-free EDs (1 study tested both standard and sugar-free EDs)
Mean dose = 389 ml (~1.5 cans)/ session Range = 250-750 ml 2 studies investigated ED + alcohol Most common EDs: Red Bull = 10 studies Celsius = 2 studies |
Heart Rate: 2/10 studies reported increase in heart rate at 30-60 min post-consumption; 1 study found decreased heart rate at 45 sec-3 min; 6/10 studies found no change in heart rate post-consumption of ED; 1 study found a decrease in heart rate variability Blood Pressure (BP): 4/4 studies found no change in BP with ED doses of 250-500 mL ECG: 1/1 study reported no arrhythmias in ECG with 750 mL ED or alcoholic ED w/ 0.4% ethanol/kg BW Aerobic endurance: 3/5 studies reported improved aerobic endurance; 1/1 study reported improved stroke volume; 1/1 study reported improved resting energy expenditure after 4 wk daily consumption Blood glucose and free FAs: 2/6 studies reported increased blood glucose, no changes in remaining studies; 3/3 studies reported no change in blood lactate but inconsistent on free FAs Body Composition: 2/4 studies reported a decrease in fat mass and % body fat in long-term follow-up of ED consumption |
|
Goldfarb et al., 2014 AMSTAR: 7/11 Systematic Review: 17 case reports: 14 studies involving 15 cases + 2 cases from authors' institution No overlap w/ Burrows 2013 (RCTs) |
Systematic review of PubMed and Embase for articles published from Jan 1980 - Feb 2013 (English, French, or Spanish) Included all cases of acute CV events potentially associated with ED w/ sufficient clinical information |
13 male cases; 15 cases <30 y, range 13-58 y 13 Men 4 Women No predisposing cardiac abnormality or previous cardiac disease (1 minor) |
Acute ingestion of >480 mg caffeine within 8 h considered “acute heavy consumption” this corresponds to >3 cans (16 oz) of several EDs in short time period Chronic heavy consumption >200 mg/ day of caffeine from EDs over >1 wk ED & Co-ingestions: Red Bull + vodka (2) GNC Speed Shot Race Energy Blast Monster + marijuana NOS Red Bull + Monster XL + MDMA 4 studies did not report ED brand (1 +vodka) Range caffeine intake: 80-1,600 mg |
4 cases atrial fibrillation: 13y male: 85mg caf/NR 14y male: NR mg/Red Bull 16y male: NR mg/Red Bull + vodka 58y male: 575mg/NR 1 case supraventricular tachycardia: 23y woman: 250mg/GNC Speed Shot 1 case electrophysiological changes w/out arrhythmia: 13y female: 160mg/NR 7 cases ventricular arrhythmia or cardiac arrest: Caf rang = 80-1300mg incl: Red Bull + vodka, Monster + marijuana, NOS, Race Energy Blast, and NR 4 cases ST elevation: 17-24y males 560-800 mg/Red Bull+vodka 1,600 mg XL+MDMA 160-240mg Red Bull NR mg/ NR+vodka |
Research Recommendations
- Define excessive caffeine intake and safe levels of consumption for children, adolescents, and young adults.
- Determine the prevalence of excessive caffeine intake in children and adults beyond intake of energy drinks.
- Examine the effect of excessive consumption of caffeine and energy drinks on health outcomes in both children and adults.
- Conduct observational studies to examine the health effects of alcohol mixed with energy drinks.
References included in the review
- Burrows T, Pursey K, Neve M, Stanwell P. What are the health implications associated with the consumption of energy drinks? A systematic review. Nutr Rev. 2013;71(3):135-48. PMID: 23452281. http://www.ncbi.nlm.nih.gov/pubmed/23452281.
- Goldfarb M, Tellier C, Thanassoulis G. Review of published cases of adverse cardiovascular events after ingestion of energy drinks. Am J Cardiol. 2014;113(1):168-72. PMID: 24176062. http://www.ncbi.nlm.nih.gov/pubmed/24176062.
References not included in the review
- Government of Canada. Health Canada Health Products. Food Branch. Food Directorate. Caffeine in Foods 2012 [updated 2012-02-16]. Available from: http://www.hc-sc.gc.ca/fn-an/securit/addit/caf/food-caf-aliments-eng.php
.
- Pomeranz JL, Munsell CR, Harris JL. Energy drinks: an emerging public health hazard for youth. J Public Health Policy. 2013;34(2):254-71. PMID: 23486464. http://www.ncbi.nlm.nih.gov/pubmed/23486464.
- Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011;127(3):511-28. PMID: 21321035. http://www.ncbi.nlm.nih.gov/pubmed/21321035.
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks--a growing problem. Drug Alcohol Depend. 2009;99(1-3):1-10. PMID: 18809264. http://www.ncbi.nlm.nih.gov/pubmed/18809264.
- Institute of Medicine. Caffeine in Food and Dietary Supplements: Examining Safety: Workshop Summary. Washington, DC: The National Academies Press; 2014. Available from: http://www.nap.edu/openbook.php?record_id=18607
.
- Branum AM, Rossen LM, Schoendorf KC. Trends in caffeine intake among U.S. children and adolescents. Pediatrics. 2014;133(3):386-93. PMID: 24515508. http://www.ncbi.nlm.nih.gov/pubmed/24515508.
- Terry-McElrath YM, O'Malley PM, Johnston LD. Energy drinks, soft drinks, and substance use among United States secondary school students. J Addict Med. 2014;8(1):6-13. PMID: 24481080. http://www.ncbi.nlm.nih.gov/pubmed/24481080.
- Arria AM, O'Brien MC. The "high" risk of energy drinks. Jama. 2011;305(6):600-1. PMID: 21266673. http://www.ncbi.nlm.nih.gov/pubmed/21266673.
- Centers for Disease Control and Prevention. Caffeine and Alcohol. 2014. Available from: http://www.cdc.gov/alcohol/fact-sheets/cab.htm.
- Substance Abuse Mental Health Services Administration. Drug Abuse Warning Network (DAWN), 2011: National Estimates of Drug-Related Emergency Department Visits.HHS. Publication No. (SMA) 13-4760, DAWN Series D-39 Rockville, MD2013. Available from: http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm.
- Ferreira SE, de Mello MT, Pompéia S, de Souza-Formigoni ML. Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res. 2006;30(4):598-605. PMID: 16573577. http://www.ncbi.nlm.nih.gov/pubmed/16573577.
- Wiklund U, Karlsson M, Ostrom M, Messner T. Influence of energy drinks and alcohol on post-exercise heart rate recovery and heart rate variability. Clin Physiol Funct Imaging. 2009;29(1):74-80. PMID: 19016812. http://www.ncbi.nlm.nih.gov/pubmed/19016812.